Folate plays a crucial role in human metabolism, participating in important physiological processes such as DNA synthesis, cell division, and amino acid metabolism. However, the human body cannot synthesize folate on its own and must rely on external intake.
Active folate, with its characteristic of being directly absorbed without the need for metabolism, has become a smart choice for folate supplementation. Currently, various forms of active folate salts are available on the market. But which one is the most suitable for our folate supplementation needs?
A comparative study published in the "Molecules" journal in 2021 has provided us with the answer. The study compared the stability and absorption of various active folate salts available on the market and found that 6S-5-methyltetrahydrofolate calcium C crystal form (MTHF CAC) showed excellent stability and could effectively maintain its active components. In addition, the absorption rate of MTHF CAC was also higher, making it a more effective choice for meeting the body's folate requirements.

① Challenges in the Industrialization of Active Folate
The industrialization of active folate faces several challenges, mainly including the following aspects:
I. Stability Issues
Light Exposure: Active folate can degrade under light exposure, leading to a reduction in activity. This requires strict light-avoiding measures during storage and use, increasing the cost of packaging and storage.
Humidity and Temperature: In high-temperature and high-humidity environments, the stability of active folate significantly decreases. This not only affects the shelf life of the final product but may also cause the product to lose its activity within its validity period, affecting the supplementation effect.
Storage Conditions: Due to the high sensitivity of active folate to environmental conditions, it needs to be stored in low-temperature, dry, and light-avoiding conditions. This poses higher requirements for storage facilities, increasing the storage costs in the industrialization process.
II. Complex Production Process
· High Purity Requirement: The production of active folate requires high purity to ensure its safety and effectiveness. This means that the production process must be able to effectively remove various toxic and harmful impurities, especially those that may pose a risk to human health.
· Stability Treatment: To improve the stability of active folate, specific stabilizers or special treatment methods, such as crystallization technology, need to be added to the production process to form a stable crystal form. These treatment steps increase the complexity and cost of production.
· Strict Quality Control: In the production process of active folate, the quality of each process needs to be strictly controlled to ensure the quality and activity of the final product. This requires companies to have advanced quality testing equipment and a strict quality management system, increasing production costs and management difficulties.

② Advantages of 6S-5-Methyltetrahydrofolate Calcium C Crystal Form
I. Excellent Stability
The 6S-5-methyltetrahydrofolate calcium C crystal form is produced using a unique ultrasonic crystallization process, forming a stable C crystal form. Experimental data show that the content of the active component 6S-5-methyltetrahydrofolate in C-type crystalline methyltetrahydrofolate calcium salt (MTHF CAC) hardly changes after 120 hours of storage at room temperature, while the content of active components in other methyltetrahydrofolate calcium salts (MTHF CA) and aminoglycan methyltetrahydrofolate salts (MTHF GA) significantly decreases under the same conditions, and the content of impurities such as para-aminobenzoic acid, JK12A, and 5-methyltetrahydropteronic acid significantly increases.
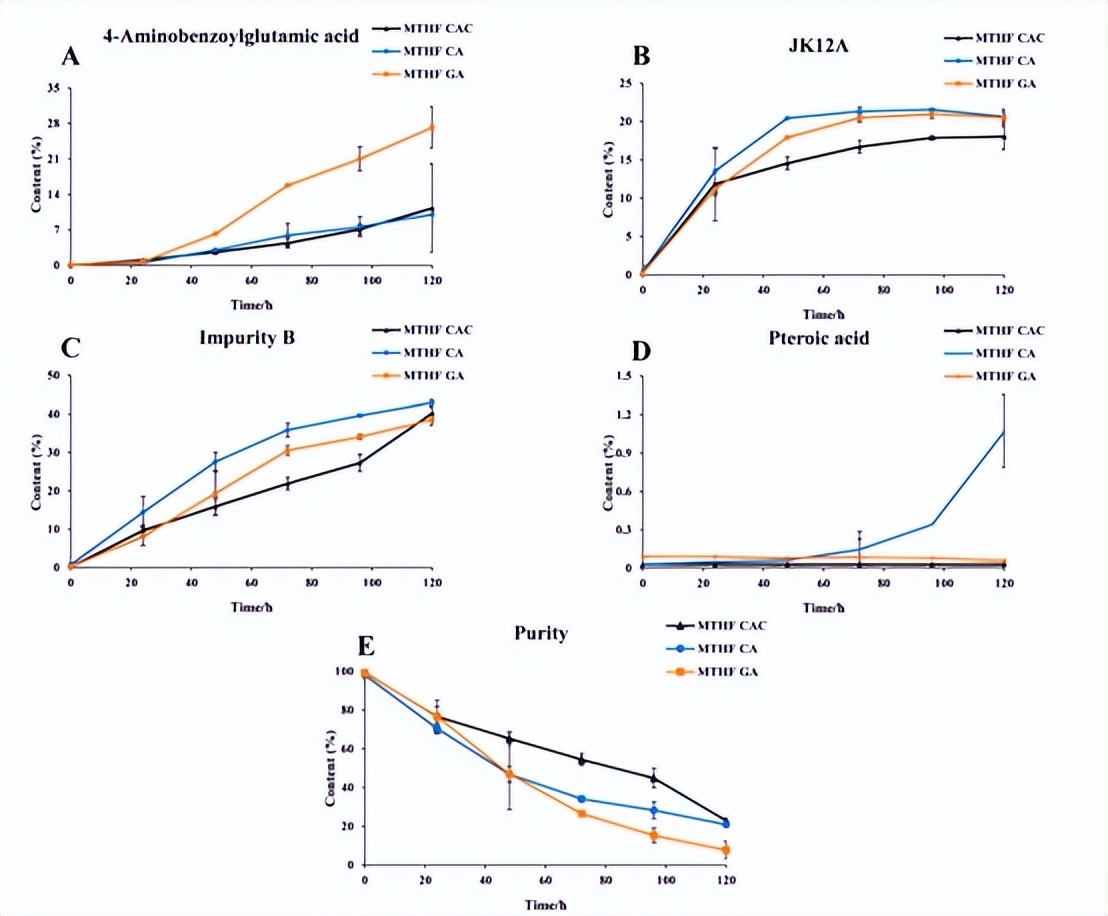
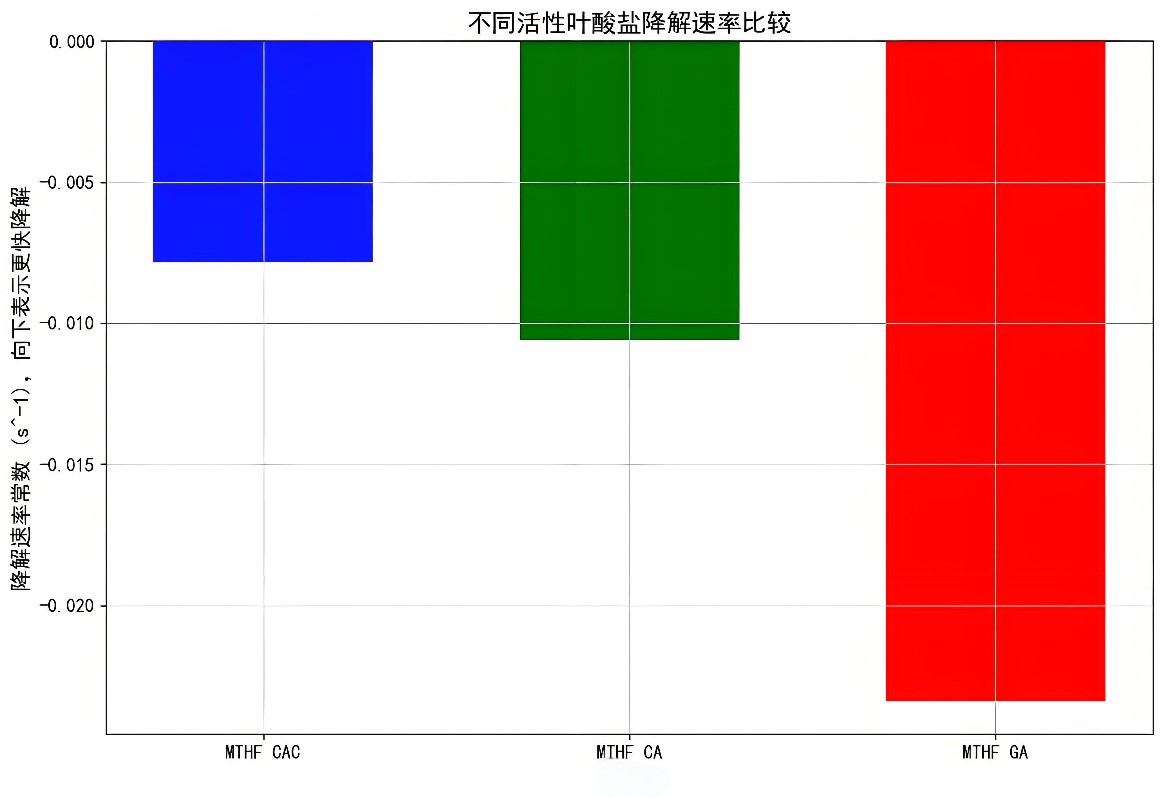
Specifically, the degradation rate of MTHF CAC within 120 hours is only 1.5%, while that of MTHF CA and MTHF GA is 10.2% and 15.6%, respectively.
Further stability tests indicate that MTHF GA degrades the fastest, followed by MTHF CA, and MTHF CAC is the most stable.
This excellent stability allows MTHF CAC to maintain its activity during storage and use, ensuring a stable folate supplement within its validity period.
II. Higher Absorption and Utilization Rate
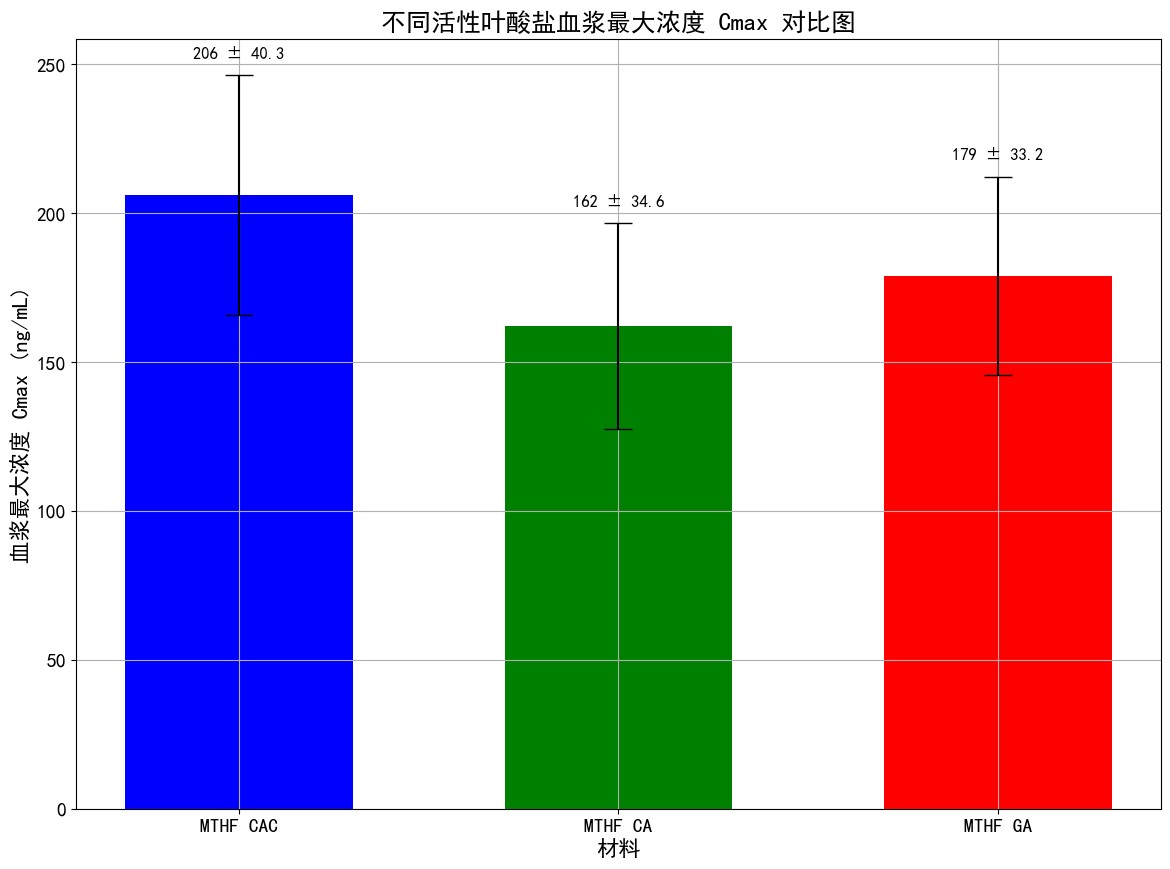
In a pharmacokinetic experiment in rats, MTHF CAC demonstrated significantly higher absorption and utilization rates.
The experimental results indicate that, after oral administration, the maximum concentration (Cmax) of 6S-5-methyltetrahydrofolate in rat plasma following MTHF CAC administration is 1.27 times higher than that of MTHF CA and 1.15 times higher than that of MTHF GA.
In addition, the area under the curve (AUC(0–t)) of MTHF CAC is 868 ± 213 h·ng/mL, which is much higher than that of MTHF CA (247 ± 67.0 h·ng/mL) and MTHF GA (399 ± 83.9 h·ng/mL).
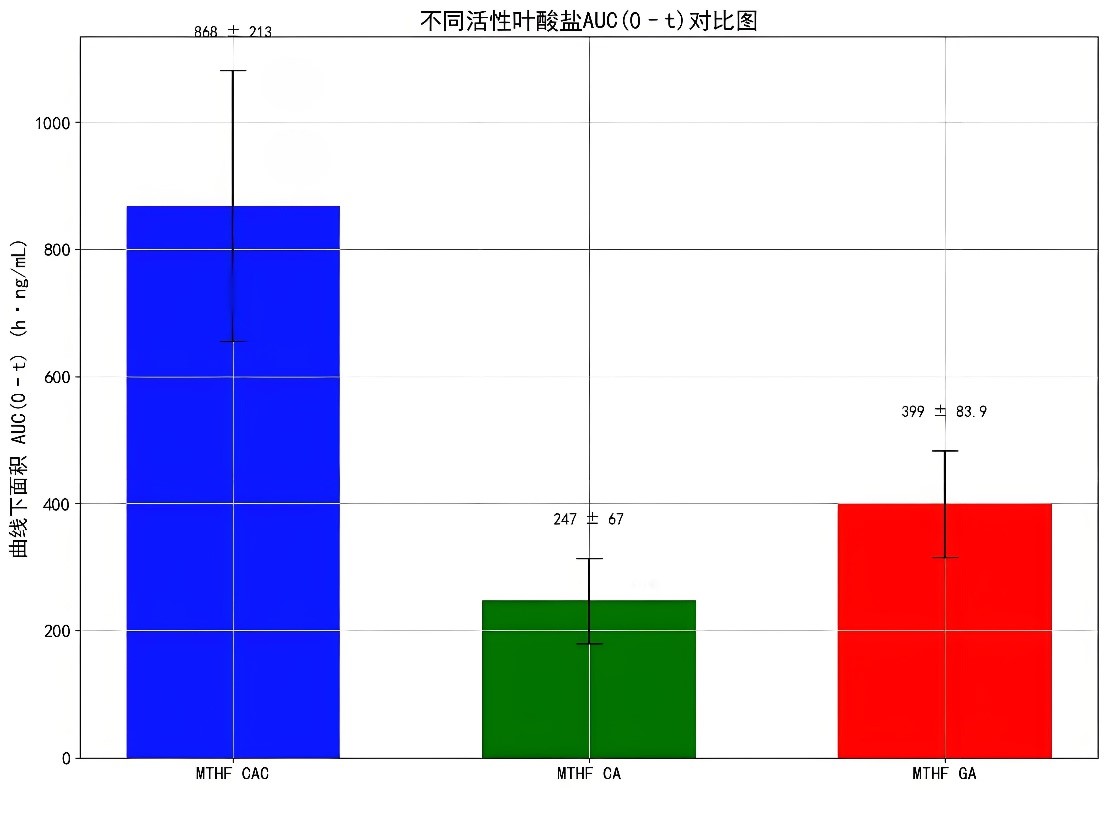
These data indicate that MTHF CAC has a higher absorption efficiency in the body, can more quickly increase the plasma folate concentration, and maintain it for a longer time.
The calculated results of relative bioavailability also confirm this. The relative bioavailability of MTHF CAC compared to MTHF CA and MTHF GA is as high as 351% and 218%, respectively.
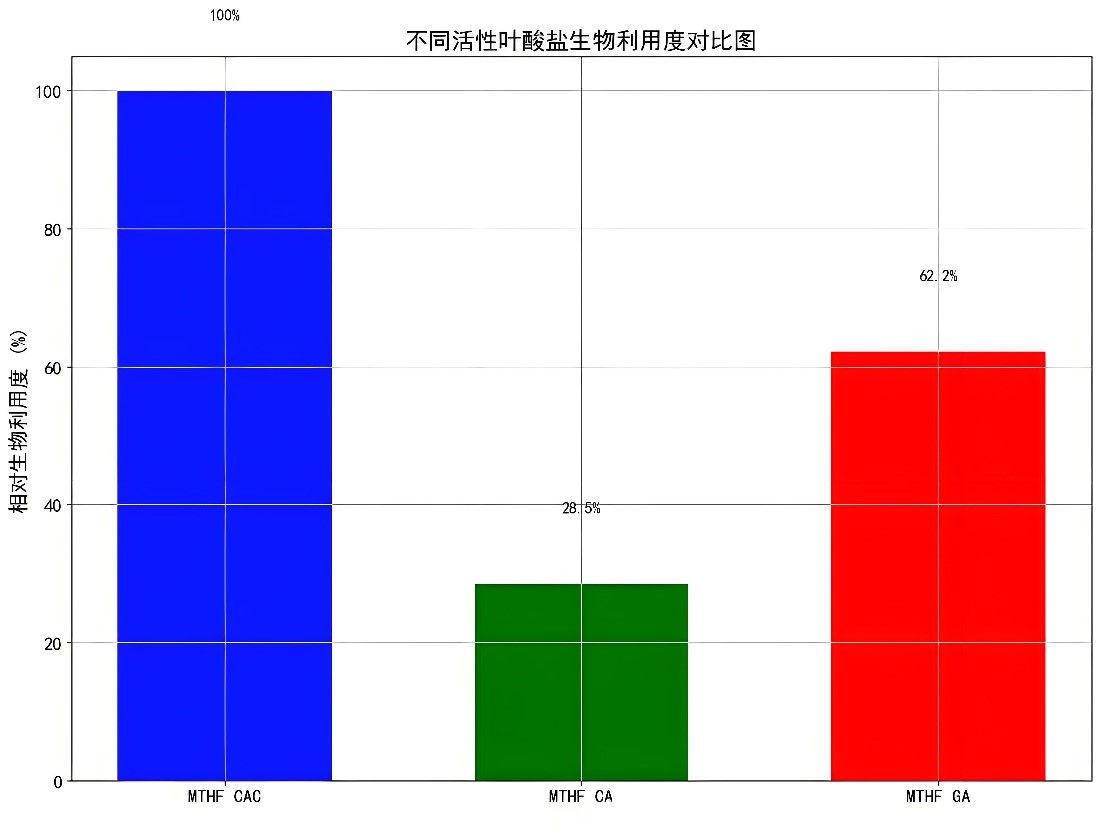
This means that at the same dose, MTHF CAC can provide more effective folate to the human body, better meeting physiological needs.
III. Longer Duration of Action
The mean residence time (MRT(0–t)) of MTHF CAC in rats is 3.7 ± 1.9 hours, while that of MTHF CA and MTHF GA is 1.0 ± 0.2 hours and 1.5 ± 0.3 hours, respectively. This result indicates that MTHF CAC has a longer duration of action in the body and can continuously provide folate to the body.
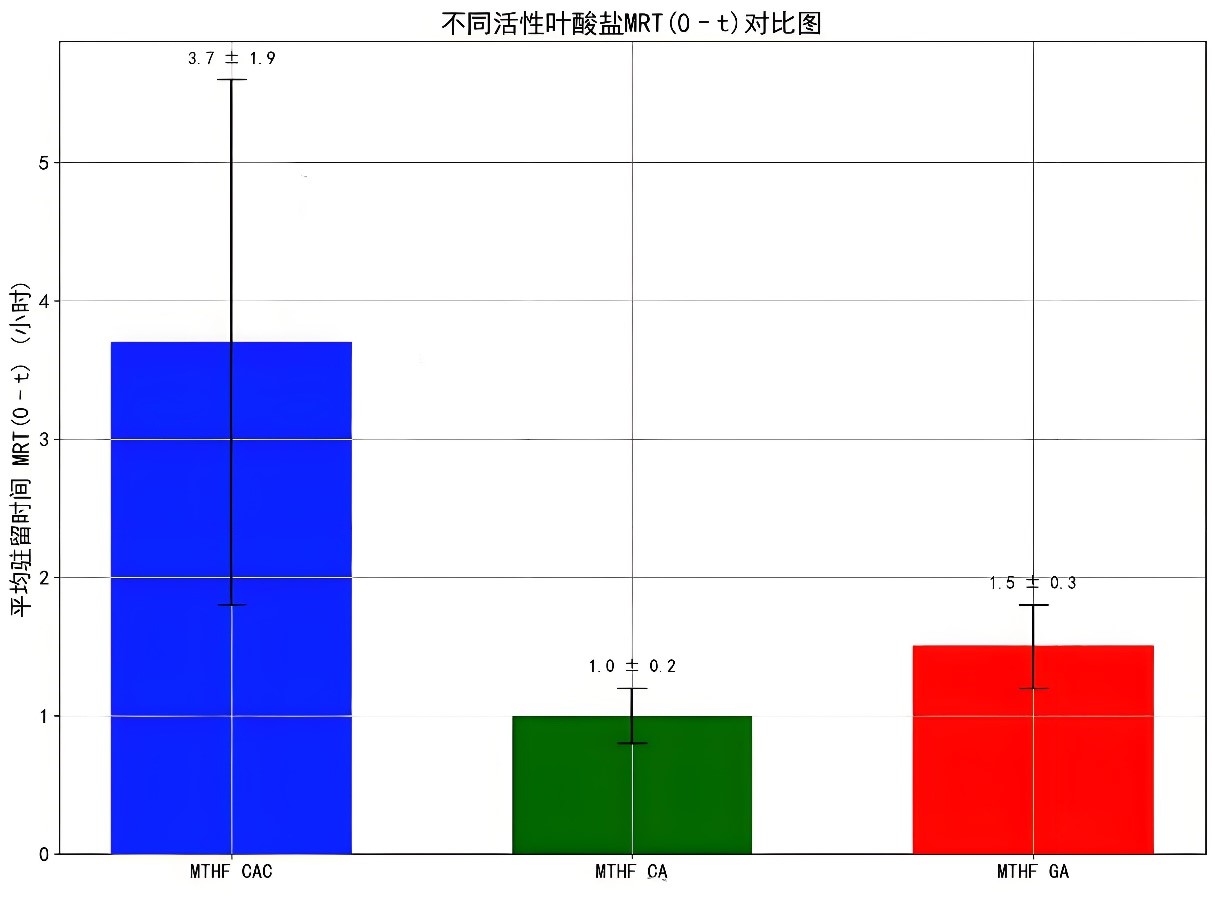
This persistence gives MTHF CAC an advantage in maintaining stable folate levels, which helps to better exert the physiological functions of folate, such as promoting DNA synthesis and maintaining normal cell division. At the same time, a longer duration of action also means that users can reduce the frequency of intake, improving the convenience of use.
These data show that the absorption and utilization efficiency of MTHF CAC in the body is significantly higher than that of other active folate salts, better meeting the body's need for folate.
③ Prospects for the Application of 6S-5-Methyltetrahydrofolate Calcium C Crystal Form
C-type crystalline 6S-5-methyltetrahydrofolate calcium salt (MTHF CAC) is a new type of folate supplement that has shown significant advantages in stability, absorption and utilization rate, and duration of action. Its excellent stability ensures the maintenance of folate activity during storage and use, and its higher absorption and utilization rate and longer duration of action make it better able to exert the physiological functions of folate in the body.
In the future, with further research on C-type crystalline 6S-5-methyltetrahydrofolate calcium salt, it is expected to further optimize its preparation process and improve its performance. At the same time, the potential of C-type crystalline 6S-5-methyltetrahydrofolate calcium salt in clinical applications is worth looking forward to, and it is expected to provide a more effective solution for the prevention of folate deficiency-related diseases.

References:
Lian, Z.; Chen, H.; Liu, K.; Jia, Q.; Qiu, F.; Cheng, Y. Improved Stability of a Stable Crystal Form C of 6S-5-Methyltetrahydrofolate Calcium Salt, Method Development and Validation of an LC–MS/MS Method for Rat Pharmacokinetic Comparison. Molecules 2021, 26, 6011.

 Español
Español Português
Português  русский
русский  Français
Français  日本語
日本語  Deutsch
Deutsch  tiếng Việt
tiếng Việt  Italiano
Italiano  Nederlands
Nederlands  ภาษาไทย
ภาษาไทย  Polski
Polski  한국어
한국어  Svenska
Svenska  magyar
magyar  Malay
Malay  বাংলা ভাষার
বাংলা ভাষার  Dansk
Dansk  Suomi
Suomi  हिन्दी
हिन्दी  Pilipino
Pilipino  Türkçe
Türkçe  Gaeilge
Gaeilge  العربية
العربية  Indonesia
Indonesia  Norsk
Norsk  تمل
تمل  český
český  ελληνικά
ελληνικά  український
український  Javanese
Javanese  فارسی
فارسی  தமிழ்
தமிழ்  తెలుగు
తెలుగు  नेपाली
नेपाली  Burmese
Burmese  български
български  ລາວ
ລາວ  Latine
Latine  Қазақша
Қазақша  Euskal
Euskal  Azərbaycan
Azərbaycan  Slovenský jazyk
Slovenský jazyk  Македонски
Македонски  Lietuvos
Lietuvos  Eesti Keel
Eesti Keel  Română
Română  Slovenski
Slovenski  मराठी
मराठी  Srpski језик
Srpski језик 








 Online Service
Online Service