I. Literature Overview
-
Title
An Open Trial of 5-Methyltetrahydrofolate in Elderly Depressed Patients - Basic Information
- Publication Date: June 1993
- Journal: Annals of Clinical Psychiatry
- Research Type: Open-label clinical trial
3. Core Conclusion
The study confirmed that oral 5-methyltetrahydrofolate (MTHF) showed significant efficacy in elderly depressed patients: among 20 patients meeting DSM-III-R diagnostic criteria, 16 completed at least 4 weeks of treatment, and 81% (13 patients) showed significant improvement in depressive symptoms (HAM-D-21 score decreased by ≥50%). No obvious drug-related adverse reactions were observed during treatment, indicating its safety and clinical application potential.

II. Detailed Research Design
-
Research Objective
To evaluate the therapeutic effect of gastric-resistant oral MTHF (50 mg/day) in elderly depressed patients and explore its feasibility as a safe intervention. - Intervention Protocol
- Dosage and Formulation: 50 mg/day gastric-resistant oral preparation (enteric coating technology to protect active ingredients for direct intestinal absorption and reduce gastric acid damage).
- Sample Characteristics: 20 elderly patients (aged 60-82 years, mean 68.5 years), all meeting DSM-III-R depression diagnostic criteria, HAM-D-21 score ≥18, and without comorbid severe physical diseases or mental disorders.
3. Research Period
1-week placebo washout period + 6-week open-label treatment, with weekly assessment of depressive symptoms (HAM-D-21) and safety indicators.
III. Research Achievements and Product Value
- Efficacy Data
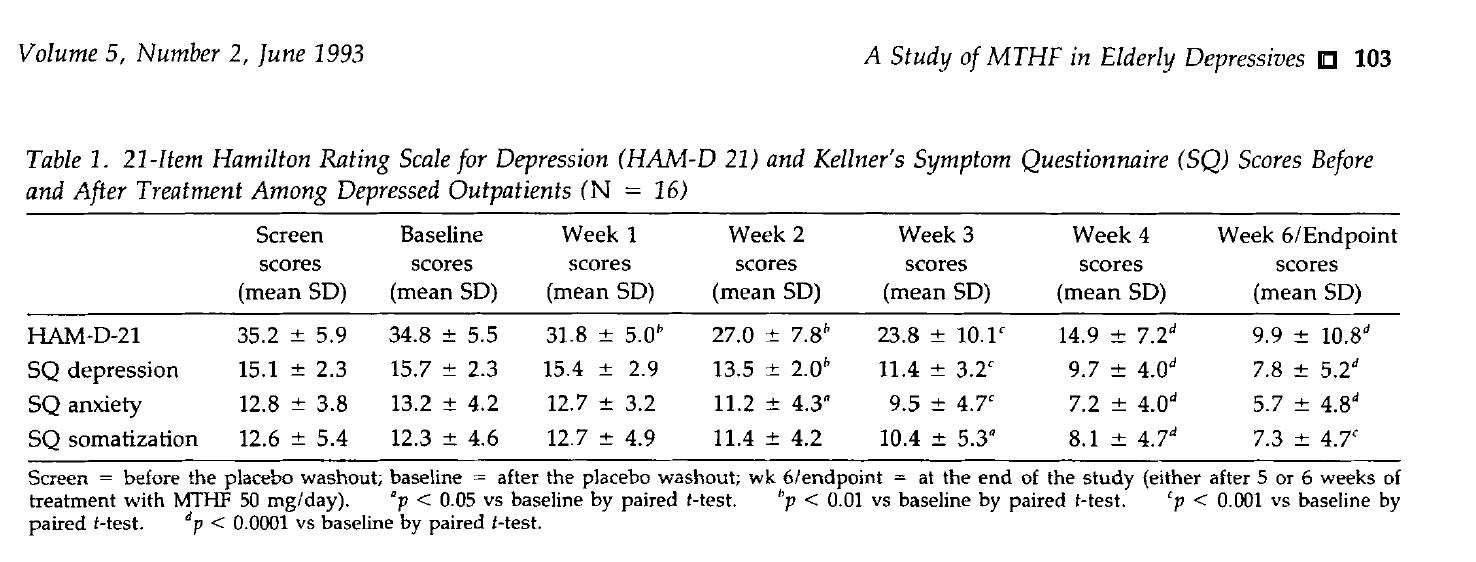
- Rapid Improvement: After 6 weeks of treatment, the HAM-D-21 score significantly decreased from baseline 34.8±5.5 to 9.9±10.8 (p<0.0001), with simultaneous remission of accompanying symptoms such as anxiety and somatization.
- High Response Rate: 81% of patients achieved clinical response (score decrease ≥50%), indicating that about 81 out of every 100 elderly patients can benefit.
- Mechanistic Advantage: MTHF participates in methylation metabolism, promotes the synthesis of S-adenosylmethionine (SAMe), and regulates the metabolism of neurotransmitters such as dopamine and 5-hydroxytryptamine, improving the pathological mechanism of depression from the root (complementary to the target of traditional antidepressants).
2. Safety Evidence
- No clinically significant abnormalities were found in laboratory indicators such as blood routine and liver/kidney functions during treatment. Only a few patients experienced mild non-specific symptoms such as headache and insomnia, without definite drug-related adverse reactions.
- Product Characteristics of Magnafolate:
- High purity: ≥99.8%, impurity JK12A <0.1%.
- High safety: Certified as "Naturalization Folate", the product reaches practically non-toxic level, without using toxic raw materials such as formaldehyde and p-toluenesulfonic acid.
- Direct absorption: No metabolism required, direct absorption, bioavailability 3-5 times higher than traditional folate.
3. Clinical Significance
- Aging-adaptive advantages: Free from cardiac toxicity and orthostatic hypotension risks of traditional antidepressants, especially suitable for elderly people with reduced liver and kidney functions.
- Combined application: Can be used as an adjuvant therapy for antidepressants or alone for mild depression and drug-intolerant patients, expanding treatment options.
IV. Mechanism Interpretation and Academic Extension
-
Mechanism of Action
As the active folate form in the body, MTHF affects depressive pathology through the following pathways:
- Methyl donor role: Participates in central nervous system methylation to maintain normal neurotransmitter synthesis, DNA repair, and cell membrane phospholipid metabolism.
- SAMe regulation: Promotes brain SAMe production, which acts as a methyl donor in the methylation modification of neurotransmitters such as norepinephrine and 5-hydroxytryptamine, regulating synaptic transmission efficiency.
- Homocysteine metabolism: Reduces plasma homocysteine levels, decreases its neurotoxic effects, and improves the cognitive-emotional regulation network.
2. Academic
Support and Evidence Chain
This study forms an evidence closed loop with subsequent studies:
- Synergy of combined medication: Passeri et al. (1991) double-blind trial showed that 15 mg/d 5-MTHF combined with antidepressants increased the HAM-D score reduction by 4.1 points compared with the monotherapy group (p<0.01).
- Breakthrough in drug-resistant populations: A 2023 clinical study (NCT not publicly available) showed that 15 mg/d 5-MTHF combined with SSRI improved symptoms in over 50% of SSRI-resistant patients, especially suitable for MTHFR gene C677T mutation carriers.
- Long-term safety verification: 5-MTHF has a bioavailability of 51%-54%, no cumulative toxicity after long-term use, and common adverse reactions are transient mild headache with an incidence <5%.
V. Core Advantages of Magnafolate Product
|
Dimension |
Magnafolate® |
Traditional Folate/Competitors |
|
Purity |
≥99.8% (HPLC detection, compliant with China National Health Commission Announcement No. 13, 2017 + USP standards) |
95%-97.9% |
|
Technical Barrier |
Patented ultrasonic crystallization technology, stable at room temperature for 48 months |
Requires low-temperature storage, poor stability |
|
Safety |
No use of toxic raw materials like formaldehyde and p-toluenesulfonic acid, controlling harmful impurities such as JK12A and 5-Methyltetrahydropteroic acid; product reaches practically non-toxic level (Shanghai CDC) |
May contain genotoxic impurities |
|
Patent Layout |
Over 40 invention patents covering crystal forms, preparation processes, and application scenarios |
Few patents |
VI. Summary of Literature Interpretation
The pioneering study in 1993 first confirmed that 5-methyltetrahydrofolate (Magnafolate) has definite efficacy and safety in geriatric depression, with its mechanism closely related to neurotransmitter metabolism and methylation networks. With high-purity raw materials and strict impurity control, Magnafolate products exhibit extremely high safety, while optimizing bioavailability and patient compliance, thus providing a low-risk and highly adaptive supplementary treatment plan for elderly depressed patients, and expected to become a new choice in clinical treatment.
Market Prospect: With the global aging aggravation, the mental health market is expected to reach $537.97 billion by 2025.
Magnafolate, with its evidence-based advantages, is expected to play important value in scenarios such as clinical nutritional support and functional health products.
References: Gian Paolo Guaraldi, Maurizio Fava, Fausto Mazzi, and Pietro la Greca. "An Open Trial of Methyltetrahydrofolate in Elderly Depressed Patients." Annals of Clinical Psychiatry 5, no. 2 (1993): 101-105. https://doi.org/10.3109/10401239309148970.

 Español
Español Português
Português  русский
русский  Français
Français  日本語
日本語  Deutsch
Deutsch  tiếng Việt
tiếng Việt  Italiano
Italiano  Nederlands
Nederlands  ภาษาไทย
ภาษาไทย  Polski
Polski  한국어
한국어  Svenska
Svenska  magyar
magyar  Malay
Malay  বাংলা ভাষার
বাংলা ভাষার  Dansk
Dansk  Suomi
Suomi  हिन्दी
हिन्दी  Pilipino
Pilipino  Türkçe
Türkçe  Gaeilge
Gaeilge  العربية
العربية  Indonesia
Indonesia  Norsk
Norsk  تمل
تمل  český
český  ελληνικά
ελληνικά  український
український  Javanese
Javanese  فارسی
فارسی  தமிழ்
தமிழ்  తెలుగు
తెలుగు  नेपाली
नेपाली  Burmese
Burmese  български
български  ລາວ
ລາວ  Latine
Latine  Қазақша
Қазақша  Euskal
Euskal  Azərbaycan
Azərbaycan  Slovenský jazyk
Slovenský jazyk  Македонски
Македонски  Lietuvos
Lietuvos  Eesti Keel
Eesti Keel  Română
Română  Slovenski
Slovenski  मराठी
मराठी  Srpski језик
Srpski језик 









 Online Service
Online Service