Folate is an essential nutrient for the human body. Active folate (6S-5-methyltetrahydrofolate, 5-MTHF), known for its high absorption rate and safety, has become a crucial source of folate supplementation. However, recent studies have revealed that 5-MTHF may generate an oxidation byproduct called JK12A during production and storage, which may pose potential health risks.

What is JK12A?
JK12A is one of the primary oxidation byproducts of 5-MTHF. Research indicates that although 5-MTHF can be directly utilized by the human body, its stability is poor, and it is prone to oxidation to form JK12A under conditions such as light exposure, high temperatures, or alkaline environments. The potential toxicity of JK12A requires careful attention.
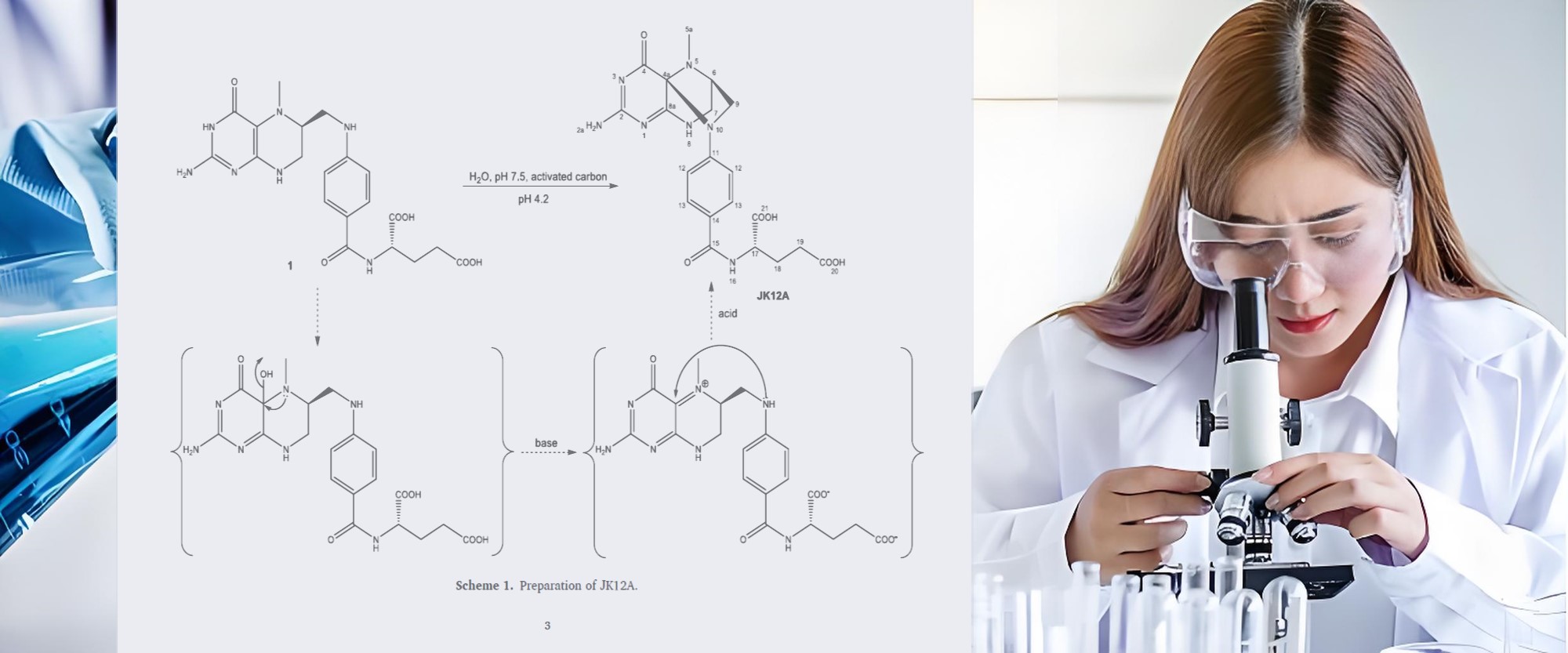
Potential Risks of JK12A:
Key Insights from Zebrafish Experiments
To evaluate the safety of JK12A, research teams conducted experiments using zebrafish embryo models, revealing the following results:
· Decreased Embryo Survival Rate: When the concentration of JK12A reached 7.04 mM, the embryo survival rate significantly decreased starting from 24 hours, and after 72 hours, the survival rate was only 50% of the control group.
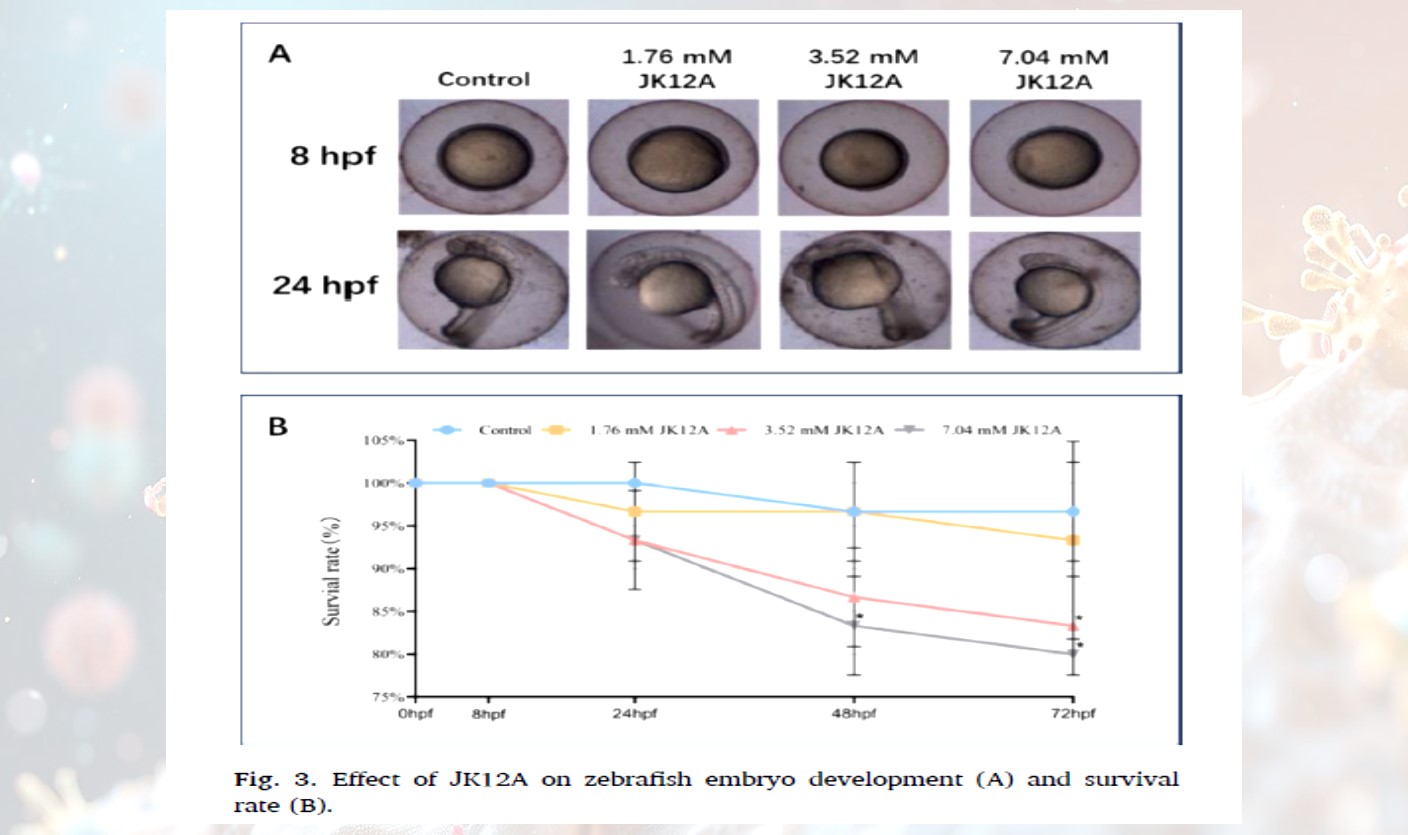
· Abnormal Heart Development: High concentrations of JK12A led to reduced heart rates in embryos, with some exhibiting pericardial edema.
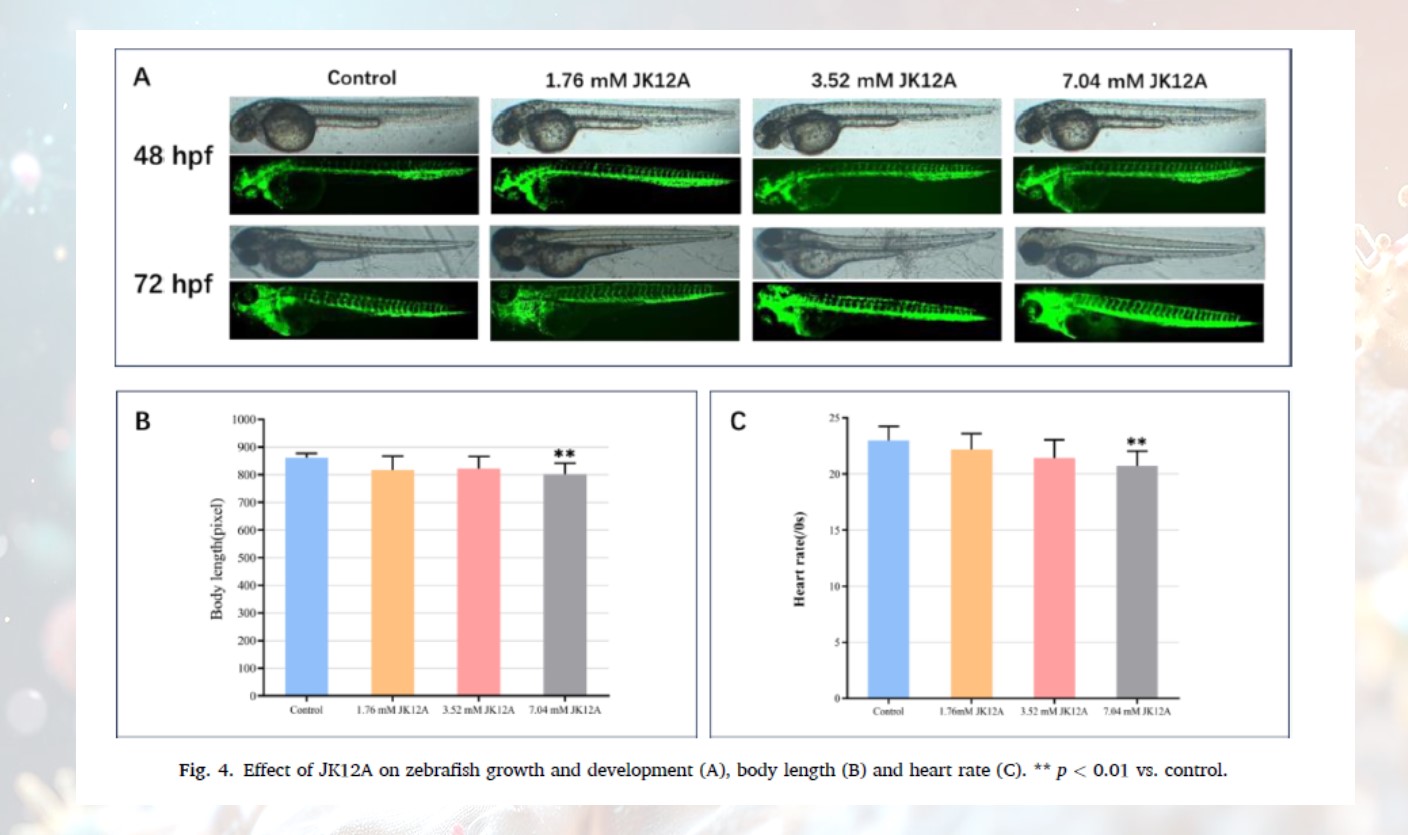
· Impeded Growth and Development: Under high-concentration exposure for 72 hours, the body length growth of zebrafish embryos decreased by 30%. The expression of key heart development genes (such as has2, hand2, and nkx2.5) was significantly downregulated, which may affect the heart's repair capacity.
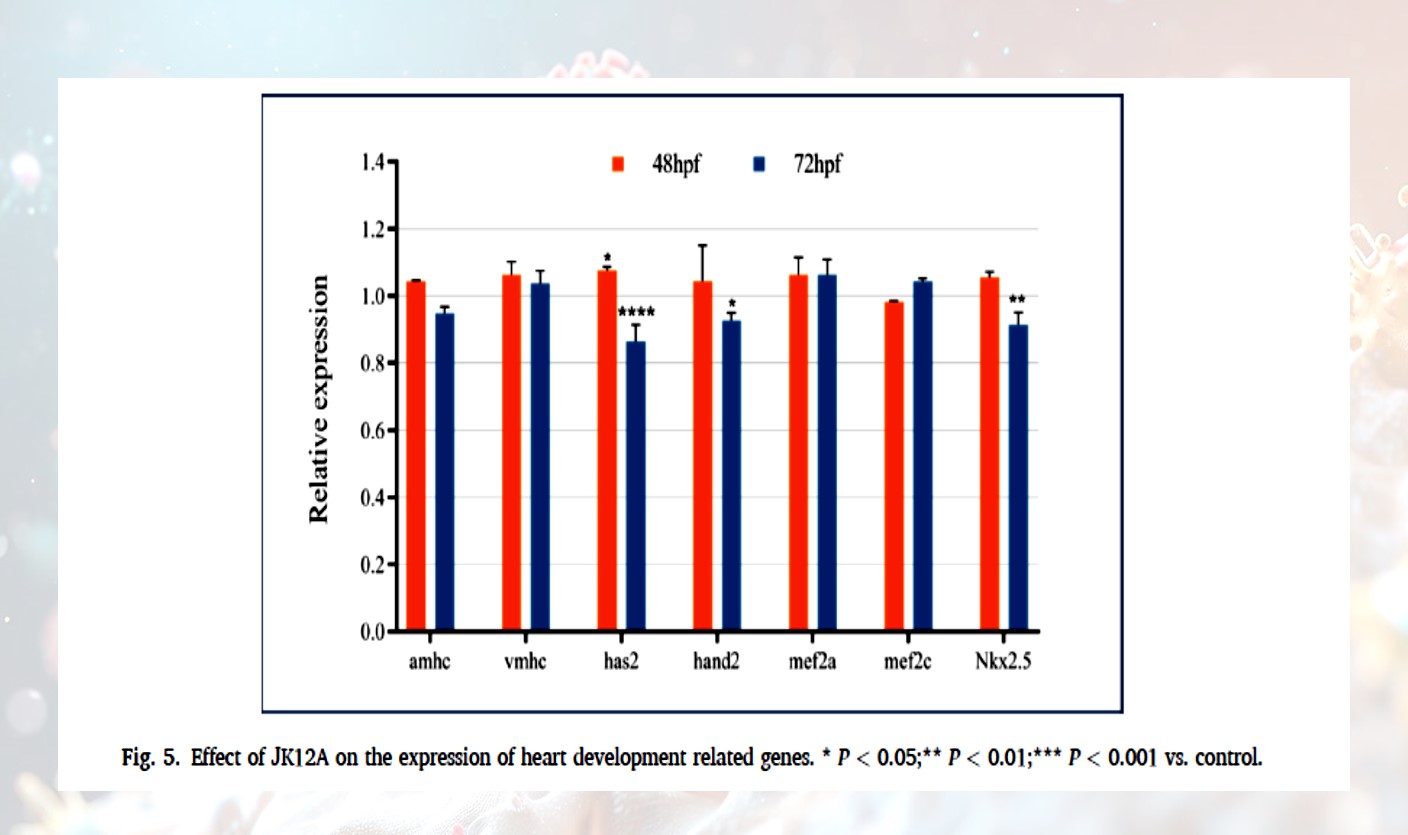
· High concentrations of JK12A exhibit significant cardiotoxicity and overall developmental toxicity, potentially interfering with the expression of heart development genes and affecting embryo development.
· Additionally, another study found that JK12A has a significant concentration-dependent inhibitory effect on the proliferation of T lymphocytes. T lymphocytes are an important component of the human immune system, responsible for identifying and eliminating pathogens and abnormal cells. The immunosuppressive effects of JK12A may weaken immune function, increasing the risk of infections and diseases, especially for vulnerable populations such as pregnant women, infants, and the elderly.

How to Prevent JK12A?
Scientific Selection of Active Folate Products
As an oxidation byproduct in the production and storage of active folate (5-MTHF), JK12A poses potential risks to embryonic development, particularly for pregnant and infant groups. Faced with a wide range of active folate products on the market, how should we make choices?
For Professionals:
· Focus on Raw Material Limit Standards: The National Health Commission's Announcement No. 13 of 2017 in China define the residual amount of JK12A should be ≤0.1%, which is one-tenth of the standard set by the United States Pharmacopeia (1.0%). Products that meet this announcement standard should be given priority.
· Evaluate Production Processes: Innovative patented production technologies can effectively suppress oxidation reactions. For example, Magnafolate, through an innovative ultrasonic crystallization process, stabilizes JK12A at below 0.1%, meeting both Chinese and American standards simultaneously.
· Verify Inspection Reports: Verify product inspection reports issued by third-party inspection institutions, with a focus on confirming whether the limits of impurities such as JK12A, 5-methyltetrahydropteroic acid, and D-isomer comply with the standards set by the National Health Commission's announcement.
· Scientific Storage: Store in a sealed manner in a cool, dry environment below 25°C, avoiding light and moisture to prevent accelerated oxidation and degradation due to high temperatures and humidity.
For General Consumers:
· You can opt for 6S-5-methyltetrahydrofolate calcium that has passed the naturalization folate certification, avoiding the risks of developmental toxicity caused by the impurity JK12A.

Conclusion: Upgrading the Concept of Nutritional Supplementation with Safety at the Core
· The discovery of JK12A serves as a wake-up call: the safety of nutritional supplements depends not only on the content of the effective ingredients but also on the strict control of risk impurities.

China has taken the lead in the quality control of active folate, strictly limiting the residual amount of JK12A to 0.1%, which is one-tenth of the American standard, providing consumers with more robust health protection.
· Maternal and infant health is no trivial matter; every effort counts and deserves our recognition and praise. Let us join hands to safeguard the health of mothers and infants.

· Reference: Wang Y. et al. Oxidation product of 5-methyltetrahydrofolate: Structure elucidation, synthesis, and biological safety evaluation. Journal of Molecular Structure. 2024.

 Español
Español Português
Português  русский
русский  Français
Français  日本語
日本語  Deutsch
Deutsch  tiếng Việt
tiếng Việt  Italiano
Italiano  Nederlands
Nederlands  ภาษาไทย
ภาษาไทย  Polski
Polski  한국어
한국어  Svenska
Svenska  magyar
magyar  Malay
Malay  বাংলা ভাষার
বাংলা ভাষার  Dansk
Dansk  Suomi
Suomi  हिन्दी
हिन्दी  Pilipino
Pilipino  Türkçe
Türkçe  Gaeilge
Gaeilge  العربية
العربية  Indonesia
Indonesia  Norsk
Norsk  تمل
تمل  český
český  ελληνικά
ελληνικά  український
український  Javanese
Javanese  فارسی
فارسی  தமிழ்
தமிழ்  తెలుగు
తెలుగు  नेपाली
नेपाली  Burmese
Burmese  български
български  ລາວ
ລາວ  Latine
Latine  Қазақша
Қазақша  Euskal
Euskal  Azərbaycan
Azərbaycan  Slovenský jazyk
Slovenský jazyk  Македонски
Македонски  Lietuvos
Lietuvos  Eesti Keel
Eesti Keel  Română
Română  Slovenski
Slovenski  मराठी
मराठी  Srpski језик
Srpski језик 








 Online Service
Online Service