“I’m six weeks pregnant, and my lab report says ‘MTHFR TT genotype.’ My doctor warned that my MTHFR enzyme activity is only 30-35 % of normal, causing homocysteine (Hcy) to pile up, which may trigger pre-eclampsia or even fetal loss…”
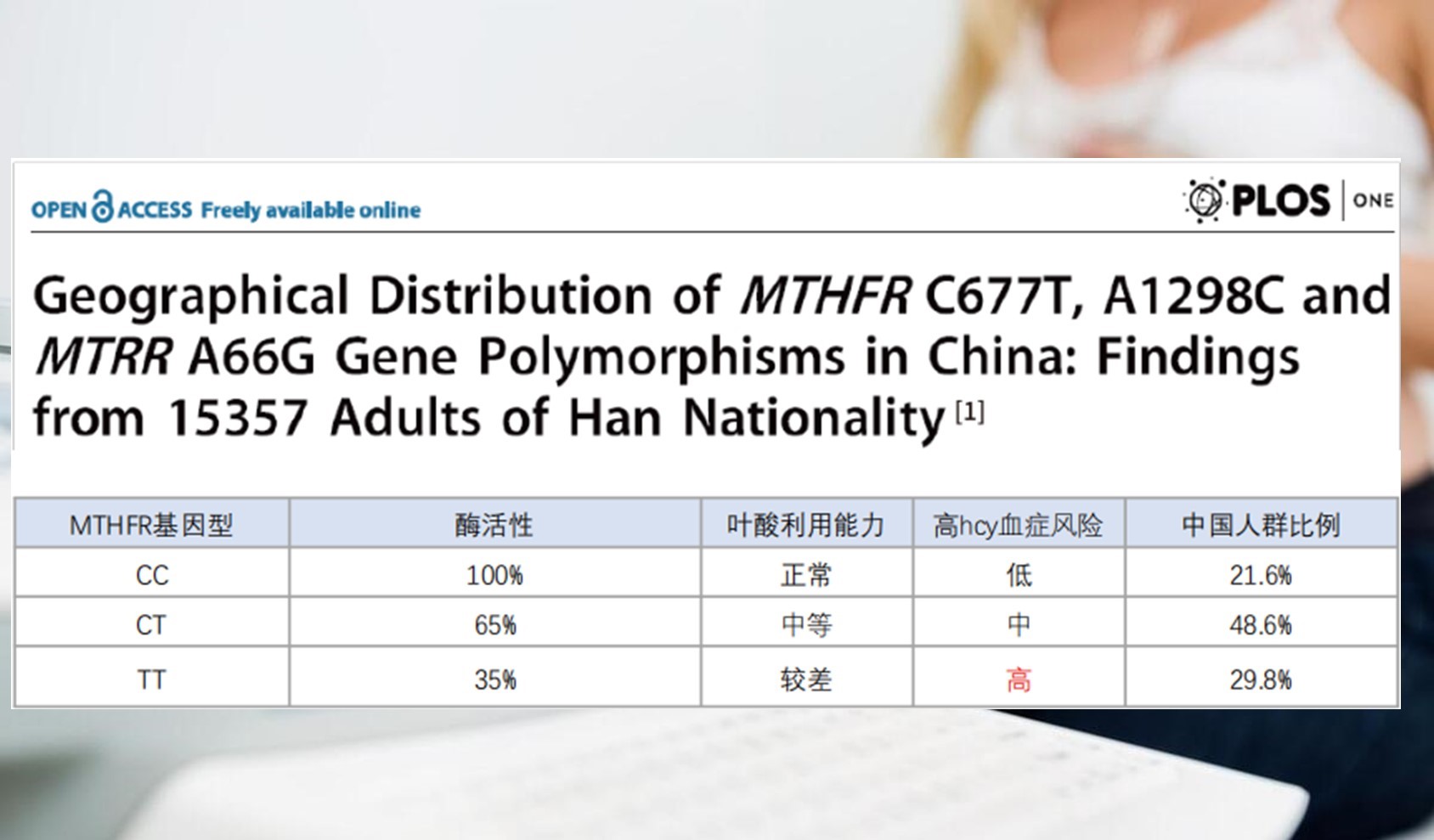
Twenty-eight-year-old Emily’s anxiety mirrors what 29.8 % of pregnant women face—a “genetic speed bump.” In every ten expectant mothers, roughly three carry the TT genotype. Far from trivial, international research has confirmed this variant acts as a bona-fide gestational risk switch.
【Evidence】How the TT genotype endangers mother and baby
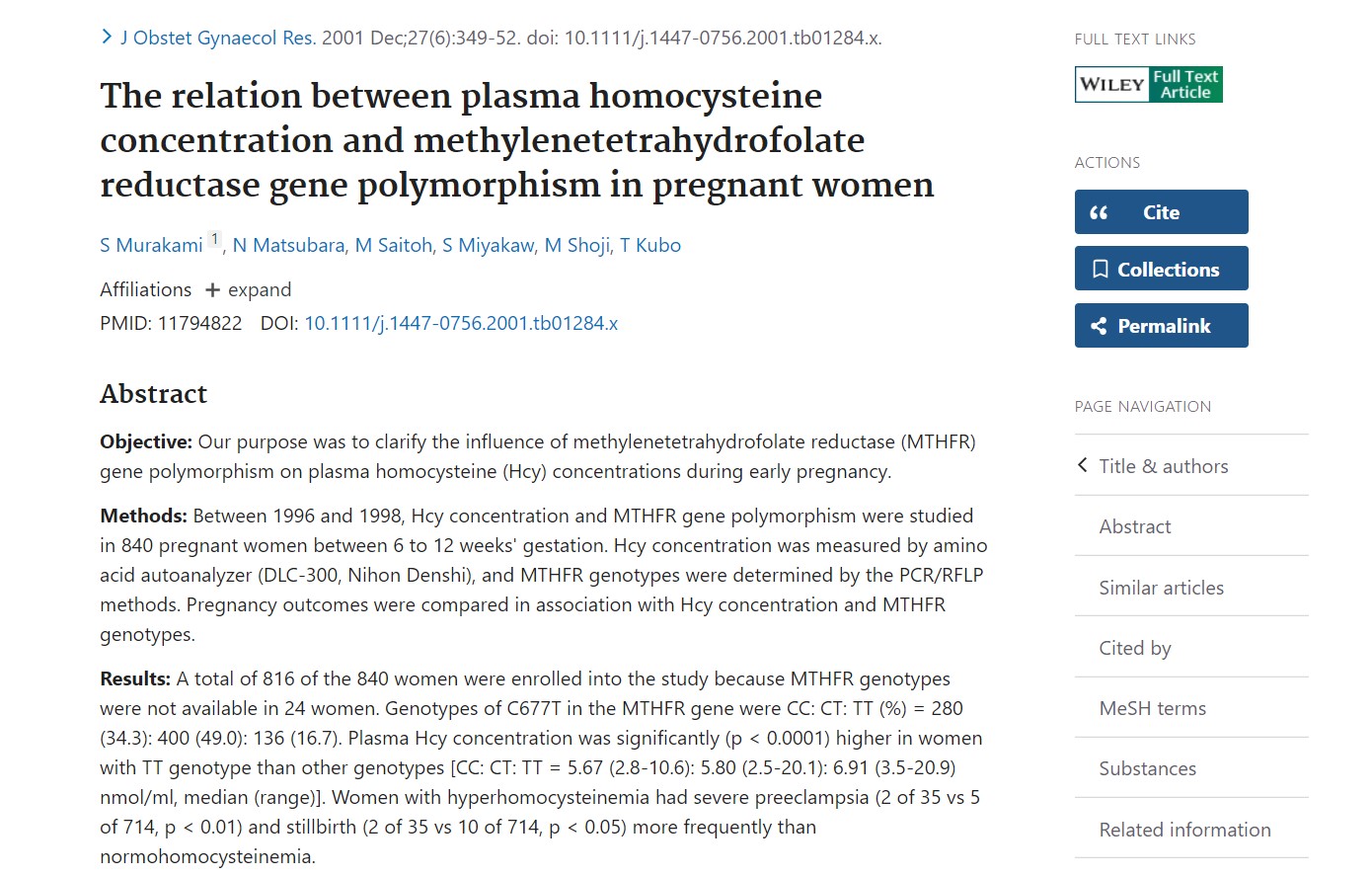
In 2002, a large Japanese cohort study (840 women, 6–12 weeks’ gestation) published in The Journal of Obstetrics and Gynaecology Research solidified the chain: TT → Hcy rise → adverse pregnancy outcomes.
I)
Median Hcy: TT 6.91 μmol/L vs CC 5.67 μmol/L and CT 5.80 μmol/L—an increase of
15–22 %.
II) Clinical sequelae: women with high Hcy had a 4-fold rise
in severe pre-eclampsia risk and a 3-fold rise in stillbirth (p < 0.01 and p
< 0.05, respectively).
Translation: the TT genotype slams the brakes on folate metabolism. Ordinary folic acid must be converted via MTHFR into active folate (6S-5-methyltetrahydrofolate). In TT carriers, enzyme activity drops 65–70 %, active folate plummets, Hcy surges and fetal and maternal safety are directly threatened.
【Solution】A three-step precision strategy
Instead of anxiety, choose science. Magnafolate® (6S-5-methyltetrahydrofolate calcium) bypasses the TT metabolic blockade at its root.

I) Skip the enzyme bottleneck
II) Magnafolate® is ready-to-use Naturalization folate. It circumvents the impaired MTHFR step, enters the bloodstream directly and rapidly lowers Hcy.
Safety first, from conception to lactation

I) Process safety: no formaldehyde, no
tosylates, no heavy metals.
II) Impurity control: 5-Methyltetrahydropteroic
acid and JK12A are kept well below regulatory limits.
III) Toxicology: rat acute toxicity MTD > 15
000 mg/kg—practically non-toxic.
V) Global approvals: US FDA GRAS (2016) + NDI 920;
Chinese National Health Commission approved for use in maternal nutrition
(2021).
Unlike synthetic folic acid, Magnafolate® is structurally identical to
circulating folate and does not accumulate as Unmetabolized Folic Acid. Safe
for the entire perinatal period and even for neonatal supplementation.
【Action Plan】What TT mothers should do now

I) Test two biomarkers: Hcy (target < 8 μmol/L) and serum vitamin B12 (deficiency worsens Hcy accumulation).
II) Choose supplements containing Magnafolate® plus a balanced B-complex.
III) Re-check every four weeks: serum folate (short-term) and erythrocyte folate (long-term), adjusting the regimen with your obstetrician.
【Cautions】
• This article is educational; individual plans
must be tailored by a qualified clinician.
• Inform your doctor if you take antiepileptics (e.g., phenytoin) or
methotrexate—both can disturb folate metabolism.
A TT genotype is not a verdict, only a reminder: switch to a folate your body can use immediately. Choose Magnafolate®—to lay a smooth “highway of life” for your baby and to give yourself calm and confidence.
References
[1] Yamada H, et al. J Obstet Gynaecol Res. 2002;28(1):21-26.
[2] Lian Z, Liu K, Gu J, Cheng Y, et al. China Food Additives, 2022(2).
(Clinical vignette is dramatized; data are real.)

 Español
Español Português
Português  русский
русский  Français
Français  日本語
日本語  Deutsch
Deutsch  tiếng Việt
tiếng Việt  Italiano
Italiano  Nederlands
Nederlands  ภาษาไทย
ภาษาไทย  Polski
Polski  한국어
한국어  Svenska
Svenska  magyar
magyar  Malay
Malay  বাংলা ভাষার
বাংলা ভাষার  Dansk
Dansk  Suomi
Suomi  हिन्दी
हिन्दी  Pilipino
Pilipino  Türkçe
Türkçe  Gaeilge
Gaeilge  العربية
العربية  Indonesia
Indonesia  Norsk
Norsk  تمل
تمل  český
český  ελληνικά
ελληνικά  український
український  Javanese
Javanese  فارسی
فارسی  தமிழ்
தமிழ்  తెలుగు
తెలుగు  नेपाली
नेपाली  Burmese
Burmese  български
български  ລາວ
ລາວ  Latine
Latine  Қазақша
Қазақша  Euskal
Euskal  Azərbaycan
Azərbaycan  Slovenský jazyk
Slovenský jazyk  Македонски
Македонски  Lietuvos
Lietuvos  Eesti Keel
Eesti Keel  Română
Română  Slovenski
Slovenski  मराठी
मराठी  Srpski језик
Srpski језик 








 Online Service
Online Service