At around eight weeks into her pregnancy, Emily began taking her prescribed folic acid tablet every day. Like many expectant mothers, she knew that folate supplementation helps prevent neural tube defects, but she had never imagined that the form of folate she took might influence the levels of certain “invisible guardians” in her breast milk.

Recently, a study published in the European Journal of Clinical Nutrition caught the attention of the nutrition community.
A research team in Vancouver, Canada enrolled 60 pregnant women between gestational weeks 8 and 21, assigning them to two groups: one received the commonly used synthetic folic acid (0.6 mg/day), the other an equimolar dose of (6S)-5-methyltetrahydrofolic acid (5‑MTHF, 0.625 mg/day).
After 16 weeks of supplementation, some participants continued until one week after delivery. At that point, researchers collected their breast milk and analyzed both folate forms and 19 human milk oligosaccharides (HMOs).
HMOs are unique carbohydrates found in breast milk—over 150 varieties exist. Although infants do not directly absorb them, HMOs play essential roles in cultivating a healthy gut microbiome and training the immune system. Among them, 3′‑sialyllactose (3′SL) is particularly notable for helping babies resist inflammation and infection.
While it has been known that HMO composition is influenced by genetics and certain modifiable factors such as maternal nutrition, the specific impact of folate supplementation remained unclear—until now.
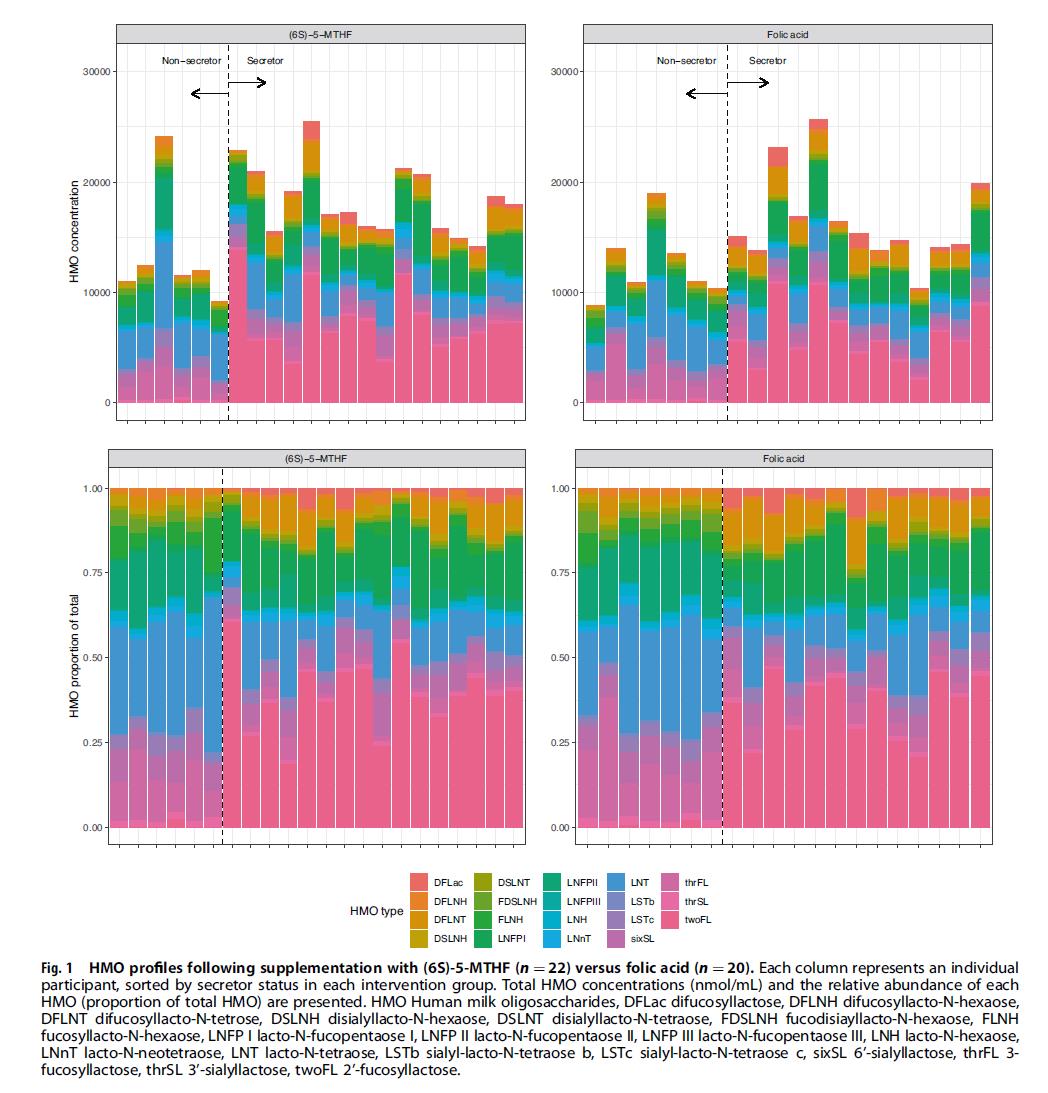
An unexpected finding emerged from this study: although total HMO levels did not differ significantly between the two groups, the proportion of Unmetabolized Folic Acid (UMFA) in the breast milk of the folic acid group reached as high as 29%, more than ten times that of the 5‑MTHF group. More notably, data analysis revealed that higher UMFA levels were associated with lower total HMO concentrations and significantly reduced 3′SL.
This suggests that prolonged intake of synthetic folic acid leading to UMFA accumulation could quietly diminish the levels of these beneficial components in breast milk—a signal worth noting for a baby’s long‑term health.
So, is there a way to meet prenatal folate needs while avoiding the potential pitfalls of UMFA?
The 5‑MTHF group in the study points to one answer.

Because (6S)-5‑MTHF is the active reduced form of folate, it requires no metabolic conversion in the body and therefore does not generate UMFA. In breast milk from this group, reduced folate forms accounted for about 98%, with UMFA virtually undetectable.
This form is also known commercially as Magnafolate, a naturalized folate produced without harmful substances such as formaldehyde or p‑toluenesulfonic acid, achieving a safety profile classified as practically non‑toxic. Magnafolate offers expectant mothers and infants a more reassuring approach to folate supplementation.
[Magnafolate® is supplied solely as an active folate raw material and does not directly provide diagnosis or treatment advice to consumers; any supplementation decisions must be made under professional medical guidance.]
References
- Lian Zenglin, Liu Kang, Gu Jinhua, Cheng Yongzhi, et al. Biological characteristics and applications of folate and 5‑methyltetrahydrofolate. China Food Additives, 2022, Issue 2.
- Titaley CR, et al. Human milk oligosaccharide composition following supplementation with folic acid vs (6S)-5-methyltetrahydrofolic acid during pregnancy and mediation by human milk folate forms. Eur J Clin Nutr. 2024; DOI:10.1038/s41430-024-01476-x.

 Español
Español Português
Português  русский
русский  Français
Français  日本語
日本語  Deutsch
Deutsch  tiếng Việt
tiếng Việt  Italiano
Italiano  Nederlands
Nederlands  ภาษาไทย
ภาษาไทย  Polski
Polski  한국어
한국어  Svenska
Svenska  magyar
magyar  Malay
Malay  বাংলা ভাষার
বাংলা ভাষার  Dansk
Dansk  Suomi
Suomi  हिन्दी
हिन्दी  Pilipino
Pilipino  Türkçe
Türkçe  Gaeilge
Gaeilge  العربية
العربية  Indonesia
Indonesia  Norsk
Norsk  تمل
تمل  český
český  ελληνικά
ελληνικά  український
український  Javanese
Javanese  فارسی
فارسی  தமிழ்
தமிழ்  తెలుగు
తెలుగు  नेपाली
नेपाली  Burmese
Burmese  български
български  ລາວ
ລາວ  Latine
Latine  Қазақша
Қазақша  Euskal
Euskal  Azərbaycan
Azərbaycan  Slovenský jazyk
Slovenský jazyk  Македонски
Македонски  Lietuvos
Lietuvos  Eesti Keel
Eesti Keel  Română
Română  Slovenski
Slovenski  मराठी
मराठी  Srpski језик
Srpski језик 








 Online Service
Online Service